Epa Design Manual Odor Corrosion Control 800
Introduction to Air Pollution - Pollution Control Technology For Air Pollution Control View Slides on & Introduction Before the introduction of air quality regulations the use of air pollution control technology was to satisfy the requirements of good engineering practice. At present the purpose of applying and/or developing a control technology is to meet ambient air quality standards and other source related regulations.
A control technology can only be applied to a controllable source. Therefore, it will be difficult to think of controlling emissions from a volcano. The cost of removing a pollutant from a source generally increases exponentially with the percentage of control. However, this relationship will change if it is possible to recover the pollutant for some economic purposes. For example, the removal of sulfur from gas processing plants was economically feasible in 1960’s because of the high price of sulfur in world market. Application of control technology requires knowledge of source, effluents from the source, air pollution regulations and waste generated from the technology.
Some times it is possible to develop a successful air pollution control technology which leads to the problem of disposing the waste. The techniques for controlling air pollution can be either without an air pollution control device or with air pollution control equipment. The general methods for techniques without an air pollution control device include process change, change in fuel, improve dispersion, good operating practices, and plant shutdown/relocation.
Control equipment remove the pollutant, convert to less harmful contaminant or recover a valuable material for further use. Techniques without Emission Control Devices Process Change: This technique involves a modification of an existing process or the introduction of a new process. Since 90’s the process change has been known as “pollution prevention”. Consider the example of painting operations in automobile industry. Large quantities of volatile organic compounds and hazardous air pollutants were released as a result of these operations.
After the passage of 1990 CAAA many operations have substituted water based, low HAP paints for oil based paints for reducing emissions. Some operations have implemented processes such as electro-deposition, dip tank and powder coating. Process modification is a popular technique to control air pollution. Major efforts are underway in all industries to modify processes to reduce pollution. For example, new oxy furnaces are being developed by Libbey Owens Ford in Toledo to reduce ozone emissions during glass manufacturing. Changes in industrial processes to reduce raw materials and fuels also lead to reduction in air emissions. The promotions to use fluorescent lights and to conduct energy audits by electric utilities are examples of less electricity demand.
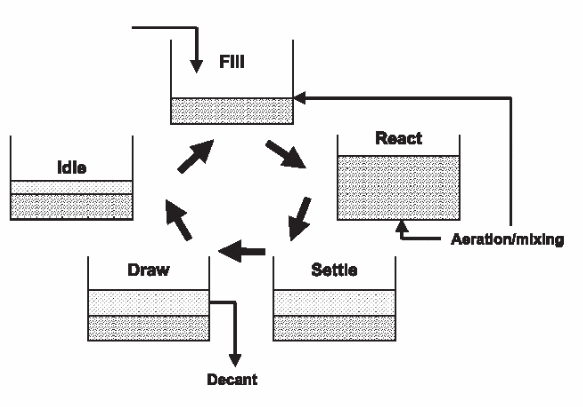
EPA 625/1-80-012. DESIGN MANUAL. ONSITE WASTEWATER TREATMENT. AND DISPOSAL SYSTEMS. ENVIRONMENTAL PROTECTION AGENCY. System to the groundwater must be control 1ed, the two treatment-di sposal. Filters, although odors from open filters receiving septic tank effluent. No matter how well a plant is designed and operated, there is the potential for accidents to happen. Accidents can be as minor as small spills.
This results in less fuel use at the power plant and hence less air pollutants are emitted. The use of wind energy, geothermal energy, hydroelectric power and solar energy is increasing and is helping in reducing air pollution. The US EPA has initiated a major effort in the area of pollution prevention. The purpose is to “prevent” pollution rather than control it at the point of release.
The goal is not to produce solid or hazardous waste. Change in Fuel: This technique involves the use of less polluting fuel to reduce air pollution. Use of low sulfur fuel instead of high sulfur fuel by electric utilities is an example of this method. Remember that low sulfur fuel is much more expensive than high sulfur fuel. The other choice for an electric utility can be the use of natural gas as a fuel. Fuel switching based on meteorological conditions or air pollution forecasts have been used to prevent air pollution problem in many areas.
The following table lists the advantages and disadvantages of a number of alternative fuels: Source: US EPA Fact Sheet Use of oil with low ash content or natural gas for a dryer at an asphalt plant to reduce particulate matter is another example of this method. Introduction of compressed natural gas, propane, ethanol and oxygenated fuels for automobiles have helped in the reduction of air pollutants in the U.S. The use of natural gas in North America and Europe for winter heating brought significant improvement in air quality in most cities. Nuclear power plants are relatively pollution free when compared to the coal fired power plants.
However, they have been subjects of controversy in their overall environmental impact. Improve Dispersion: This approach is based on the concept that dilution of air contaminants before they reach ground will lower the concentrations to which the population is exposed. The use of this approach for industry is discouraged by the US EPA. However, local and state agencies use the concept to develop air pollution control strategies for their area.
Before the 1970 CAAA the most widely approach to air problems was based on the motto Dilution is the solution to pollution. This is evident from the tall stacks build by Tennessee Valley Authority and others in 60’s and 70’s. Another form of this approach is practiced in the form of intermittent control or an air quality prediction system. This approach attempts to control source emission rate during the periods of high ground level concentrations. Production curtailments, a plant shutdown, fuel switching, or other strategies achieve the reduction in source emission rate. The critical periods are determined from weather related data in the area.
The prediction systems are based on observations, predictive equations or a combination of observations and predictions. The first documented predictive intermittent control system was placed in 1941 at the lead-zinc smelter at Trail, British Columbia (Canada). The plant is located in a valley near the US-Canada border. The crop damage to orchards in the US from the nighttime emissions brought to ground during morning inversion breakup led to the formation of an international tribunal. The study led to the development of an intermittent control system to focus on the period from 3 AM to three hours after sunrise during growing season.
TVA developed an AQPS for Paradise, Kentucky power plant. Several hours before the adverse meteorological conditions are predicted, the power output is reduced to prevent violation of NAAQS.
Many cities in Canada and US have regulations to curtail industrial and other activities during times of observed poor air quality. Alberta Environment asks industry to cut production during early morning hours of poor air quality in City of Calgary and Edmonton. Many western mountain communities in US issue a public notice to shut off wood stoves when PM10 levels exceed certain value. The use of oxygenated gasoline in winter months in many cities across US is an example of a national scale intermittent control for CO emissions. High CO emissions are observed in many communities during winter months. Ozone action days are declared during summer months to avoid violations of NAAQS based on observations of meteorological variables (e.g.: temperature) and ground level ozone concentration. A procedure to identify ozone action days in Toledo during 1997 is given in.
Good Operating Practices: Release of unnecessary air pollutants could be avoided by maintaining good housekeeping in the plant and performing proper maintenance. For example, liquid chemicals spread over the floor evaporates rapidly and will cause an increase in emission rate for that chemical. Plant Shutdown/Relocation: This is not a pleasant solution for a community because of economic impact. Most cities develop land-use planning and industrial zoning regulations to avoid this situation.
Encouragement to use new technology through tax credits or grants may help a company to relocate the plant within the city. In some cases to shutdown the plant is only viable solution. Use of Control Devices The emissions from the plant are passed through a control device before releasing to atmosphere. The pollutants are removed, destroyed or transformed in the control device before discharging into ambient air. The devices are discussed in the following sections.
General public thinks of these devices whenever they think about air pollution control. However, there are other methods available to reduce air pollution as discussed above. You should consider other possibilities before deciding to use a control device. Related Internet Site Site includes information on air control systems, monitoring equipment and other services. Control of SOx The control of SO 2 is largely based on chemical means.
The sulfur present in organic compounds can be converted to various forms by oxidation or reduction. Sulfur oxidizes to Sulfur Dioxide (SO 2) and then Sulfur Trioxide (SO 3). In the atmosphere SO 3 reacts with water to form sulfuric acid, which then reacts with ammonia or other cat-ions to form particles of ammonia sulfate or some other sulfate. These small particles are responsible for urban particulate and visibility problems.
Reduction means the removal of oxygen or the addition of hydrogen. The major source of SO 2in the US are coal burning electric power plants. The typical SO 2 content of the emissions is about 0.1% SO 2 or 1000 ppm. Low concentration of SO 2 in gas stream makes it unprofitable for recovery as H 2SO 4.
The most widely used method is scrubbing. The installation and operation of these air pollution control device is expensive and require large capital expenditure. The scrubbing or flue gas desulphurization (FGD) processes can be classified as (i) Throwaway or regenerative processes or (ii) wet or dry processes. The major FGD processes are: Limestone scrubbing Lime scrubbing Dual Alkali processes Lime-spray drying Wellman-Lord process The SO 2 is removed by inducing exhaust gases to react with a chemical absorbent as they pass through a tower.
Limestone Wet Scrubber This is a widely used device for removing SO 2. The incurring exhaust gas after the removal of solid fly ash particles is passed to a tower. A limestone (CaCO 3) slurry is sprayed on the incoming exhaust gas.
The SO 2 dissolves in the slurry and reacts with limestone producing CO 2 and solid CaSO 3. SO 2 + CaCO 3 + H 2O >CaSO 3 + H 2O + CO 2. During the development phase of this technology during 1970s and early 1980s, the problems of corrosion, solid deposition, scaling and plugging, mist eliminator plugging, poor reagent utilization and poor solid-liquid separation were encountered. Most of these problems have either been eliminated or reduced to manageable size through proper design and careful operation. The limestone scrubbers are still expensive and troublesome. The large amount of solid waste produced remains a disposal problem. Lime Scrubber The process is similar to limestone wet throwaway process described above.
Lime (CaO) is used instead of limestone. Lime hydrates to Ca(OH) 2 in the hold tank and is sprayed on the exhaust gases. Ca(OH) 2 is more chemically reactive than limestone. During the process CaSO 3 is produced. SO 2 + CaO+ H 2O >CaSO 3 + H 2O Dual Alkali Scrubber This is a wet regenerative system.
Two reagents are used to remove SO 2. The scrubbing is done by sodium sulfite or sodium hydroxide.
Lime-spray Drying The exhaust gases react with a fine slurry mist of lime. The heat of the exhaust gases is used to dry the reacted slurry into calcium sulfite particles. SO 2 + CaO >CaSO 3 The particles are captured in a particle collection device along with other particles in the plant. The removal efficiency of SO 2 in a dry scrubbing system is typically 70%. The system is 15 to 30 percent cheaper to install and operate as compared to a conventional wet scrubbing system. The waste products are easier to handle.
Control and Treatment of VOC and Hydrocarbons Control and treatment of VOC and organic hazardous air pollutant emissions are generally accomplished by adsorption, incineration, condensation and gas absorption. The methodology is usually chosen depending upon the temperature, composition and volumetric flow rate of the emission stream, space constraints and allowable installation and operational costs.
A brief description of each method is given below: Adsorption: This is one of the most commonly used methods, especially for controlling emissions from small sources. It can be physical adsorption or chemisorptions.
The later is rarely used for the VOC emission control because, it involves a less-reversible chemical bonding of the adsorbate (pollutant) and the adsorbing solid ( packing) and is relatively expensive. Physical adsorption uses the Van der Waals force, giving the advantage of reversibility and regeneration due to the weaker bonding of the gas and adsorbent material. The adsorbed material can be either recovered or incinerated.
Regeneration is usually accomplished by heating or extraction/displacement. Activated carbon is a commonly used adsorbent because of its high surface area and material hardness. It has between 800 and 1200 m 2/g of surface area.
In general, activated carbon and other adsorbents such as hollow aluminum spheres coated with a catalyst can be employed in a fixed, moving or fluidized bed system. Fluidized bed systems, though more expensive to build and operate, yield high contacting with low pressure loss and regeneration can be accomplished within the system. The fixed beds are less expensive and provide longer packing life, but provide less contacting per unit length and require a larger pressure loss; because they are regenerated individually. Moving beds have properties between fixed and fluidized beds. The useful life of activated carbon can be determined using break through curves. Regeneration can be achieved by contact with a hot, inert gas, contact with a low pressure gas stream and pressure reduction over the bed. Steam desorption is the most commonly used process for regeneration.
Incineration: Incineration or combustion is another common VOC control technology. Complete combustion or oxidation of pure hydrocarbons produces carbon dioxide and water.
Sulfur and nitrogen compounds produce acid gases and limited air supply results in the formation of carbon monoxide. Complex organic compounds may not oxidize completely in the residence time and ash may form. Most VOC oxidation must be done at high temperature, unless catalysts are involved. Flares, thermal oxidizers and catalytic converters all use oxidation chemistry to treat VOC emissions. Flares mostly treat moderate to high temperature concentrations. All of the heat produced by the combustion process is lost when the flares are used. Most thermal oxidizers treat emission streams with maximum VOC concentrations of 25% of the LEL ( lower explosive limit).
Catalyst beds, especially when used to enhance the oxidation of VOCs (usually noble metals like platinum and palladium) must be able to withstand high temperatures and must be designed so that a minimum pressure drop is created when the gas passes through the bed. For example by using catalytic converters, thermal oxidation of the by-products of the incomplete engine combustion can be safely accomplished at temperatures much lower than would be required without the aid of catalysis. Condensation: Condensation and gas absorption are most commonly used for highly concentrated VOC streams that are advantageous to recover and the relatively large expense is warranted. It employs a drop in temperature and/ or increase in pressure to cause the VOCs in the emission stream to condense. The cleaned air stream is separated from the condensate containing target pollutants. In many cases, very large temperature drops are required to achieve effective condensation, requiring significant energy investment to accomplish cooling.
Condensation is used to recover gasoline and fuel vapors at gasoline loading terminals and in gasoline dispensing facilities. It is also used in the adsorbent regeneration process to separate solvents from the stream to separate solvents from the stream used to regenerate the activated carbon. Gas Absorption: Gas absorption involves the absorption of a gas into a liquid. Water can be used for recovery of water-soluble compounds such as acetone and low molecular weight alcohols, which can later be separated from water using distillation. Additives are often used to increase the effective mass transfer rate of the pollutant from the gas phase into the liquid phase, affecting the surface tension, reducing interfacial resistance and increasing the apparent solubility.
Gas absorption can be expensive, however it is generally used only to recover VOCs that have a secondary market value. Gas absorption techniques are used for the recovery of a variety of chemicals in the coke manufacturing industry. They are often called scrubbers. Particulate Control The control of particulate matter is an important aspect of industrial air pollution engineering. Particles are collected by a combination of several mechanisms.
The six available mechanisms are gravitational settling, centrifugal impaction, inertial impaction, direct interception, diffusion and the electrostatic attraction. The physical phenomenon of gravitational settling, centrifugal impaction and electrostatic attraction are known to engineers. The other three mechanisms are described below. Inertial Impaction The large particles in the gas stream have too much inertia to follow the gas streamlines around the impactor and are impacted on the impactor surface, while the small particles and the gas tend to diverge and pass around the interceptor.
Direct Interception In case of direct interception, the particles have less inertia and barely follow the gas streamlines around the fiber. If the distance between the center of the fiber and the outside of the fiber is less than the particle radius, the particle will graze or hit the fiber and be 'intercepted'. Inertial impaction and direct interception mechanisms account for 99% collection of particles greater than 1 micrometer aerodynamic diameter in fabric filter systems. Diffusion In diffusion, small particles are affected by collisions on a molecular level. Particles less than 0.1 micrometer have individual or random motion. The particles do not necessarily follow the gas streamlines, but, move randomly throughout the fluid. This is known as 'Brownian Motion'.
The particles may have a different velocity than the fluid and at some point could come in contact with the fiber and be collected. Agglomeration also contributes to particle collection. There are five basic types of dust collectors in use: i) gravity settling chambers, ii) cyclones, iii) fabric filters, iv) electrostatic precipitators, and v) scrubbers. The purpose of this section is to discuss the general working of these devices.
Gravity Settling Chambers This is a simple particulate collection device using the principle of gravity to settle the particulate matter in a gas stream passing through its long chamber. The primary requirement of such a device would be a chamber in which the carrier gas velocity is reduced so as to allow the particulate matter to settle out of the moving gas stream under the action of gravity. This particulate matter is then collected at the bottom of the chamber.
The chamber is cleaned manually to dispose the waste. The gas velocities in the settling chamber must be sufficiently low for the particles to settle due to gravitational force.
Literature indicates that gas velocity less than about 3 m/s is needed to prevent re-entrainment of the settled particles. The gas velocity of less than 0.5 m/s will produce good results. Curtains, rods, baffles and wire mesh screens may be suspended in the chamber to minimize turbulence and to ensure uniform flow. The pressure drop through the chamber is usually low and is due to the entrance and exit losses.
The velocity of the particles in the settling chamber can be obtained by Stokes’ law as follows: Vs = (g( rp – r ) D 2 ) /18 µ Where, D = Diameter of the particle. G = acceleration due to gravity rp = density of the particle r = density of the gas µ = viscosity of the gas The advantages of settling chambers are: i) low initial cost, ii) simple construction, iii) low maintenance cost, iv) low pressure drop, v) dry and continuous disposal of solid particles, vi) use of any material for construction, and vii) temperature and pressure limitations will only depend on the nature of the construction material.
The disadvantages of this device are i) large space requirements and ii) only comparatively large particles (greater than 10 micron) can be collected. Because of the above advantages and disadvantages, settling chambers are mostly used as pre-cleaners. They are sometimes used in the process industries, particularly in the food and metallurgical industries as the first step in dust control. Use of settling chambers as pre-cleaners can also reduce the maintenance cost of high efficiency control equipment, which is more subject to abrasive deterioration. Cyclones: Settling chambers discussed above are not effective in removing small particles. Therefore, one needs a device that can exert more force than gravity force on the particles so that they can be removed from the gas stream. Cyclones use centrifugal forces for removing the fine particles.
They are also known as centrifugal or inertial separators. The cyclone consists of a vertically placed cylinder which has an inverted cone attached to its base. The particulate laden gas stream enters tangentially at the inlet point to the cylinder. The velocity of this inlet gas stream is then transformed into a confined vortex, from which centrifugal forces tend to drive the suspended particles to the walls of the cyclone. The vortex turns upward after reaching at the bottom of the cylinder in a narrower inner spiral. The clean gas is removed from a central cylindrical opening at the top, while the dust particles are collected at the bottom in a storage hopper by gravity. The efficiency of a cyclone chiefly depends upon the cyclone diameter.
For a given pressure drop, smaller the diameter, greater is the efficiency, because centrifugal action increases with decreasing radius of rotation. Centrifugal forces employed in modern designs vary from 5 to 2500 times gravity depending on the diameter of the cyclone. Cyclone efficiencies are greater than 90% for the particles with the diameter of the order of 10 µ. For particles with diameter higher than 20 µ, efficiency is about 95%. The efficiency of a cyclone can be increased by the use of cyclones either in parallel or in series. A brief explanation of both arrangements is given below: Multiple Cyclones: A battery of smaller cyclones, operating in parallel, designed for a constant pressure drop in each chamber. The arrangement is compact, with convenient inlet and outlet arrangements.
They can treat a large gas flow, capturing smaller particles. Cyclones in series: Two cyclones are used in series. The second cyclone removes the particles that were not collected in the first cyclone, because of the statistical distribution across the inlet, or accidental re-entrainment due to eddy currents and re-entrainment in the vortex core, thus increasing the efficiency. The advantages of cyclones are: i) low initial cost, ii) simple in construction and operation, iii) low pressure drop, iv) low maintenance requirements, v) continuous disposal of solid particulate matter, and vi) use of any material in their construction that can withstand the temperature and pressure requirements. The disadvantages of cyclones include: i) low collection efficiency for particles below 5 – 10 µ in diameter, ii) severe abrasion problems can occur during the striking of particles on the walls of the cyclone, and iii) a decrease in efficiency at low particulate concentration. Typical applications of cyclones are: i) For the control of gas borne particulate matter in industrial operations such as cement manufacture, food and beverage, mineral processing and textile industries. Ii) To separate dust in the disintegration operations, such as rock crushing, ore handling and sand conditioning in industries.
Iii) To recover catalyst dusts in the petroleum industry. Iv) To reduce the fly ash emissions. The operating problems are: i) Erosion: Heavy, hard, sharp edged particles, in a high concentration, moving at a high velocity in the cyclone, continuously scrape against the wall and can erode the metallic surface. Ii) Corrosion: If the cyclone is operating below the condensation point, and if reactive gases are present in the gas stream, then corrosion problems can occur. Thus the product should be kept above the dew point or a stainless steel alloy should be used. Iii) Build – up: A dust cake builds up on the cyclone walls, especially around the vortex finder, at the ends of any internal vanes, and especially if the dust is hygroscopic.
It can be a severe problem. Electrostatic Precipitators: Electrostatic precipitators (ESP) are particulate collection devices that use electrostatic force to remove the particles less than 5 micron in diameter. It is difficult to use gravity settlers and cyclones effectively for the said range of particles.
Particles as small as one-tenth of a micrometer can be removed with almost 100% efficiency using electrostatic precipitators. The principle behind all electrostatic precipitators is to give electrostatic charge to particles in a given gas stream and then pass the particles through an electrostatic field that drives them to a collecting electrode. The electrostatic precipitators require maintenance of a high potential difference between the two electrodes, one is a discharging electrode and the other is a collecting electrode. Because of the high potential difference between the two electrodes, a powerful ionizing field is formed. Very high potentials – as high as 100 kV are used.
The usual range is 40- 60 kV. The ionization creates an active glow zone (blue electric discharge) called the ‘corona’ or ‘corona glow’. Gas ionization is the dissociation of gas molecules into free ions. As the particulate in the gas pass through the field, they get charged and migrate to the oppositely charged collecting electrode, lose their charge and are removed mechanically by rapping, vibration, or washing to a hopper below.
In summary, the step by step process of removing particles using ESPs is: i) Ionizing the gas. Ii) Charging the gas particles. Iii) Transporting the particles to the collecting surface. Iv) Neutralizing, or removing the charge from the dust particles. V) Removing the dust from the collecting surface. The major components of electrostatic precipitators are: i) A source of high voltage ii) Discharge and collecting electrodes.
Iii) Inlet and outlet for the gas. Iv) A hopper for the disposal of the collected material. V) An outer casing to form an enclosure around the electrodes. The ESP is made of a rectangular or cylindrical casing.
All casings provide an inlet and outlet connection for the gases, hoppers to collect the precipitated particulate and the necessary discharge electrodes and collecting surfaces. There is a weatherproof, gas tight enclosure over the precipitator that houses the high voltage insulators. Electrostatic precipitators also usually have a number of auxiliary components, which include access doors, dampers, safety devices and gas distribution systems. The doors can be closed and bolted under normal conditions and can be opened when necessary for inspection and maintenance. Dampers are provided to control the quantity of gas. It may either be a guillotine, a louver or some such other device that opens and closes to adjust gas flow.
The safety grounding system is extremely important and must always be in place during operation and especially during inspection. This commonly consists of a conductor, one end of which is grounded to the casing, and the other end is attached to the high voltage system by an insulated operating lever. The precipitator hopper is an integral part of the precipitator shell and is made of the same material as the shell.
Since ESPs require a very high voltage direct current source of energy for operation, transformers are required to step up normal service voltages to high voltages. Rectifiers convert the alternating current to unidirectional current. Types of electrostatic precipitators: There are many types of ESPs in use throughout the world. A brief description of three different types is given below: A) Single stage or two stage: In a single stage ESP, gas ionization and particulate collection are combined in a single step. An example is the “Cottrell” single-stage precipitator. Because it operates at ionizing voltages from 40,000 to 70,000 volts, DC, it may also be called a high voltage precipitator. It is used extensively for heavy duty applications such as utility boilers, large industrial boilers and cement kilns.
In the two-stage precipitator particles are ionized in the first chamber and collected in the second chamber. For example, “Penny”– the two stage precipitator uses DC voltages from 11,000 to 14,000 volts for ionization and is referred to as a low voltage precipitator. Its use is limited to low inlet concentration, normally not exceeding 0.025 grains per cubic feet.
It is the most practical collection technique for many hydrocarbon applications, where the initial clear exhaust stack turns into a visible emission as vapor condenses. B) Pipe type or Plate type: In the pipe type electrostatic precipitators, a nest of parallel pipes form the collecting electrodes, which may be round, or square. Generally the pipe is about 30 cm in diameter or less. Most commonly a wire with a small radius of curvature, suspended along the axis of each pipe, is used.
The wires must be weighted or supported to retain proper physical tension and location, electrically insulated from the support grid and strong enough to withstand rapping or vibration for cleaning purpose. The gas flow is axial from bottom to top. The pipe electrodes, may be 2-5 m high. Spacing between the discharge electrode and collecting electrode ranges from 8-20 cm. Precipitation of the aerosol particles occurs on the inner pipe walls, from which the material can be periodically removed by rapping of pipes or by flushing water. The pipe type precipitator is generally used for the removal of liquid particles.
In the plate type precipitators the collection electrodes consist of parallel plates. Flashlight For Nokia N86 Free Download. The discharge electrodes are again wires with a small curvature. Sometimes square or twisted rods can be used. The wires are suspended midway between the parallel plates and usually hang free with a weight suspended at the bottom to keep them straight. Discharge electrodes are made from non-corrosive materials like tungsten, and alloys of steel and copper.
The gas flow is parallel to the plates. The plates may be 1-2 m wide and 3-6 m high. The parallel plates should be at equally spaced intervals (between 15 and 35 cm). The collection of the aerosols takes place on the inner side of the parallel plates.
The dust material can be removed by rapping either continuously or periodically. The dust particles removed fall into the hopper at the base of the precipitator. Collection electrodes should have a minimum amount of collection surface, bulking resistance, resistance to corrosion and a consistent economic design. Plate type precipitators are horizontal or vertical, depending on the direction of the gas flow. Gas velocities are maintained at 0.5-0.6 m/s in these precipitators. They’re used for collection of solid particulate. C) Dry and Wet Precipitators: If particulate matter is removed from the collecting electrodes, by rapping only, it is known as a dry precipitator.
If, on the other hand, water or any other fluid is used for removal of the solid particulate matter, then it is known as a wet precipitator. In general, wet precipitators are more efficient. However, it is the dry type plate precipitators that are predominantly used.
Efficiency: Generally, the collection efficiency of the electrostatic precipitator is very high, approaching 100%. Many installations operate at 98 and 99% efficiency. Some materials ionize more readily than others and are thus more adapted to removal by electrostatic precipitation. Acid mists and catalyst recovery units have efficiencies in excess of 99%.
However, for materials like carbon black, which have very low efficiencies due to very low collection capacity, by proper combination of an ESP with a cyclone, very high efficiencies can be achieved. The gas entering the ESP may be pre-treated (i.e., removing a portion of particulate) by using certain mechanical collectors or by adding certain chemicals to the gas to change the chemical properties of the gas to increase their capacity to collect on the discharge electrode and thus increase the efficiency. The factors affecting the efficiency of electrostatic precipitators are particle resistivity and particle re-entrainment. Both are explained below: A) Particle Resistivity: Dust resistivity is a measure of the resistance of the dust layer to the passage of a current.
For practical operation, the resistivity should be 10 7 and 10 11 ohm-cm. At higher resistivities, particles are too difficult to charge. Higher resistivity leads to a decrease in removal efficiency. At times, particles of high resistivity may be conditioned with moisture to bring them into an acceptable range.
If the resistivity of the particles is too low,( 4N 2 + 6H 2O 2NO 2 + 4 NH3+ O 2 ----->3N 2 + 6H 2O Selective catalytic reduction catalyst is best at around 300 too 400 oC. Typical efficiencies are around 80%. The selective noncatalytic reduction process involves the following: At Higher temperatures (900-1000 oC), NH3 will reduce NOx to nitrogen without a catalyst. At NH3: NOx molar ratios of 1:1, to 2:1, about 40 - 60% reduction is obtained. SNR is cheaper than SCR is terms of operation cost and capital cost. Tight temperature controls are needed. At lower temperatures, non reacted ammonia is emitted.
At higher temperatures the ammonia is oxidized to NO. The electron beam radiation process is as follows: This treatment process is under development, and is not widely used. Work is underway to determine the feasibility of electron beam radiation for neutralizing hazardous wastes and air toxics. Irradiation of flue gases containing NOx or SOx produce nitrate and sulfate ions. The addition of water and ammonia produces NH4NO3, and (NH4)2SO4 The solids are removed from the gas, and are sold as fertilizers. The staged combustion process to control NOx can be explained as under: Staged combustion processes significantly reduce NOx emissions. In the initial stage of combustion, the air supplied to the burners is less than the amount required to completely burn the fuel.
During this stage, fuel bound nitrogen is released but cannot be oxidized, so it forms stable molecules of harmless molecular nitrogen (N2). Other components of the fuel are also released without being fully oxidized. These include carbon particles and carbon monoxide. By adding a second stage, in the air-fuel mixture, the carbon and carbon monoxide can be burned, converting them to carbon dioxide.
Modifying existing coal furnaces to achieve a staged combustion process has resulted in a 30% to 50% reduction in NOx emissions. Besides reducing NOx emissions, limiting the air during the combustion process increases the efficiency of converting fuel to usable heat. This is a cheap approach.
However, it requires a larger firebox for the same combustion rate and it is difficult to get complete burning of the fuel in the second stage, so that the amount of unburned fuel and/or carbon monoxide in the exhaust gas is increased. Some practical examples of the application of this technology are: 1. Pulverized Coal Burner: A coal burner design based on staged combustion may reduce NOx by as much as 85%. The burner produces a fuel-rich primary combustion zone and controls the fuel-air mixing. These conditions lead to preferential conversion of the nitrogen in the coal to molecular nitrogen (N2). In conventional burners, this fuel nitrogen is the primary source of NOx. Additional air is introduced from the periphery of the burner to complete combustion in a secondary zone.
The design also results in low levels of carbonaceous emissions consistent with high energy efficiency. Residential Oil Furnaces: A primary innovation in the residential oil furnace was used to remove a controlled amount of heat from the fire box and thus reduce the formation of thermal NOx by almost 65%. Oil consumption was also reduced by an average of 15%. Thus fuel savings have been achieved while simultaneously protecting the environment. Small-scale industrial boilers: In boilers used for light industries and for heating large buildings, reducing the amount of oxygen available during the initial combustion stage has been demonstrated to be a viable technique to reduce NOx emissions from these boilers.
However, this results in incomplete combustion so that the amount of carbon particles emitted in the exhaust increases. The development of a burner for these boilers will limit the NOx emissions while maintaining the high efficiency of the boiler and preventing the formation of the carbon particulate. Catalytic emission control in motor vehicle exhaust: A special 3-way catalytic converter consists of a catalyst that causes nitric oxide to oxidize the carbon monoxide and hydrocarbons.
In this process, molecular nitrogen, carbon dioxide and water vapor are released. In order to make this reaction work efficiently, the proportions of NO, CO and HC entering the catalytic converter must be carefully controlled. This is done by regulating the ratio of air and fuel in the combustion chamber.
Too much fuel results in increased CO and HC emissions. Too much oxygen results in increased emissions of nitrogen oxides. An oxygen sensor in the exhaust manifold allows control, while an active feedback device adjusts the mixture of and fuel in the carburetor or fuel injection system.
CASE HISTORY A Study of Odor Control in Livestock Buildings INTRODUCTION All over the world, feed cattle markets have expanded dramatically in recent years. Odor control is one of the major issues with such livestock markets.
The issue is of considerable interest to residents as well as to owners, if such buildings (which require large initial investment to develop) are located close to a residential area. In this study, the problem of annoying odors coming from a livestock show pavilion is considered.
Up to 1,000 head of cattle are to be housed in the pavilion. The problem is further complicated by the fact that odor nuisance occurs whenever animal wastes are allowed to decompose. It must be kept in mind that 1,000 head of cattle produce 2.6 tons of solid waste and 1.0 tons of liquid waste in a day [1]. ANALYSIS OF ODORS The analysis of odors from a livestock show pavilion requires an understanding of the factors influencing them. In the following sections we will discuss odors, characteristic of animal wastes, odor nuisance from animal wastes, physical conditions in the area, etc. Odors [2,3,4,5] Odor is that property of a substance which affects the sense of smell; a human response to the chemical structure of molecules when those molecules contact the sensory surfaces of the human body. The ability to perceive an unlimited number of odor stimuli is unique among human senses and remains a biological mystery.
The response to odor varies from person to person. Humans tend to relate their odor likes and dislikes to pleasant or unpleasant past experiences. However, for certain volatile substances there are little debate---they 'stink.' The odor is determined by human odorimetry, since no other measurement method is yet available. Characteristic of Animal Wastes [6,7,8] The physical and chemical properties of animal wastes are affected by the particular characteristics of the animal, the feed ration and the environment.
The size of the animal, as measured by its live weight, is perhaps the most important parameter. The sex and breed of the animal affects the manure properties.
The digestibility of the feed ration, the fiber and protein content and the nature of other feed elements determine the composition of the excreta. The quality of the feed influences, not only the amount the animal eats daily, but also the chemical composition of the waste. Proteins, which contain most of the nitrogen of the feed, vary in digestibility, depending on the source of the protein. The nitrogen of the undigested protein is excreted in the solid feces, whereas the nitrogen of the digested proteins is absorbed and is later excreted in the urine, except for a portion, which is used to build up the flesh in the animal. Other components of the feed include potassium, phosphorus, antibiotics, etc. If large amounts of antibiotics are given to the animal then a portion will pass through the digestive tract and could severely inhibit or at times limit the biological treatment of the manure. Bearing in mind that animal wastes are unstable, biodegradable materials and that, depending on the way they are handled and stored, they may undergo rapid changes in composition, estimated average chemical compositions of different types of wastes are given in the following table.
Odor Nuisance from Animal Wastes [6,7,9] If the animal wastes accumulate and are allowed to putrefy anaerobically in the building, the odor problem will be very serious. Using a rough estimate similar to that of Curtis [9], the approximate amount of pollutant gases for 1,000 head of livestock is Ammonia (NH 3) 50,000 liters Carbon Dioxide (CO ) 2 90,000 liters (Respiratory CO 2 is not included) Hydrogen Sulfide (H 2S) 20,000 liters Methane (CH 4) 125,000 liters These data are based on the assumption that heat, water vapor and oxygen are supplied and standard atmospheric pressure and temperature are maintained. Now one can appreciate the need of odor control for a livestock show pavilion, located close to a residential area. Table: Composition of typical animal wastes [6] PARAMETERS AVERAGE CONCENTRATION (mg/l) SWINE POULTRY BEEF DAIRY BOD 5 30,800 45,500 31,300 8,350 Total Solids 34,400 118,500 81,500 24,600% Volatile 66 63 79 64 COD 70,800 156,000 316,000 50,400 Nitrogen Free Ammonia (NH 3) Nitrite (NO 2) Nitrate (NO 3) Total 3,130 1.33 1.47 5,700 - - - 7,460 856 2.7 3.1 2,650 256 2.1 1.2 585 Phosphorous (PO 4) 3,780 8,350 3,410 655 Moisture Content (%) 96 88 92 98 The characteristics of various gases are: Ammonia (NH 3) is a colorless gas with a very characteristic pungent odor.
It is much lighter than air, and low concentrations are irritating to the eyes, nose and throat. Generally, in decomposing animal wastes, it is the first gas to be noticed. Suffocation may follow inhalation of large quantities. Carbon Dioxide (CO 2) is a colorless, odorless gas. It acts as a simple asphyxiant, and because its density is greater than that of air, it is particularly dangerous in manure pits. Hydrogen Sulfide (H 2S) is a colorless gas which is heavier than air and possesses the characteristic odor of rotten eggs. It is both an irritant and an asphyxiant and is very poisonous.
Low concentrations of 1 to 2 ppm are easy to detect by smell, but higher concentrations rapidly destroy the sense of smell. Prolonged exposure to levels of 500 ppm or higher can cause death. Methane (CH 4) is a colorless, odorless gas lighter than air. If its concentration is sufficiently high it may form explosive mixtures in air. There are many other compounds like mercaptans, amines, etc, which are present in small amounts and may cause odor nuisance. Physical Conditions In the prevention and control of odors, it is essential that careful consideration be given to meteorologic, climatologic and topographic conditions in the area.
This point is discussed, in detail, at the end of the paper. Transportation System Loading and unloading zones for the transportation of animals must be located inside the building. This step will prevent the odor from animals, if any. Ventilation System [10] The purposes of a good ventilation system are: (a) removal of surplus heat emitted by the animals, if any, (b) removal of air pollutant and humidity, (c) must bring sufficient air to meet oxygen. A ventilation system must be flexible enough to cope with the seasonal changes, e.g. In winter, one will be more interested in removing water vapor produced which does not require much air while in summer, surplus heat must be removed and great amounts of air are to be drawn through the building to maintain the temperature. There are three main parts in a ventilation system: Inlet System Outlet System Regulating System.
Inlet system: This is the most important part of a ventilating system. This system handles fresh air in proper amounts, with proper velocity while the outlet system creates the necessary negative pressure in the building. This requires adjustable air inlets.
The wooden valve furnished with a sloping cover adjusted manually or automatically is the best type of inlet valve. The sloping cover gives the proper direction for the air while the adjustment of cover provides the proper air velocity. Outlet system: The system consists of electric ventilators, with adjustable airflow valves, placed in the roof. These ventilators must have a total capacity equal to 250 m 3 of air per heat producing unit in summer while 50 m 3 of air heat producing unit in winter. Regulating system: The adjustment of the ventilation system can be done manually. The manual adjustments are not preferred because of obvious dangers of wrong adjustment, e.g.
By a wrong guess about the weather. In automatic adjustment systems, small servomotors are provided to open and close the inlet and outlet valves.
Thermostats control the servomotors. The environmental requirements for 1,000 cattle as follows. Total volume requirement 20,000 cum Floor dimensions (60 * 30) sqm Temperature (indoor) 10-20 oC Maximum relative humidity 85-70% Inlet velocity (air inlet) 1.8 to 2 m/s Outlet ventilators capacity 250 cum of air per head (summer) Outlet ventilators capacity 50 cum of air per head (winter) ODOR CONTROL From the previous discussions, one can conclude that the basic measures to be taken for the control and removal of odor are (apart from proper transportation system, ventilation system, etc.): To prevent the accumulation and decomposition of excreta, i.e. Removal and treatment of animal wastes.
To prevent the possibility of diseases in animals by infection by controlling the inlet air. Finally, to treat the air vented from the building to make sure that the concentration of gases present, are not causing nuisance to the residents of the area. Removal and Treatment of Animal Wastes [7.8,12] The eventual disposal of the waste is the key for odor control because it defines the legal requirements for the degree of treatment. Since the livestock show pavilion is close to a residential area, the treatment must stabilize the manure so as not to create nuisance conditions. The wastes may be removed mechanically or by hydraulic means. The mechanical means are preferred as opposed to hydraulic means, which require the flushing of water (periodic or continuous).
The mechanical removal of the wastes is normally done with tractors, manure spreaders, or with permanently installed equipment, such as shuttle conveyors, floor augers or various pumps. The most common type of pumps used in manure handling are centrifugal, auger, diaphragm and vacuum. Augers and diaphragm pumps have been found satisfactory both for conveyance and for elevation of animal wastes, which contained no bedding [8]. The treatment of animal wastes may be classified as physical, chemical and biological.
The physical treatments are mainly storing or drying the wastes. Since the livestock pavilion is near a residential area, the drying treatment is better than storing. The reasons are that the method stabilizes the manure to some degree, reduces the weight considerably and makes the manure unattractive for fly breeding. As the drying process by spreading the manure in thin layers is not suited for our case (because of residential locality and climate), the artificial drying or dehydration is recommended. A study of these processes is done by Ludington [12]. Only incineration has been investigated out of various chemical processes. This process is drawing considerable attention because it is not only the treatment but also is final disposal but is not economically feasible even for large production units [12].
Use of lagoons is the most popular method in the category of various biological treatments currently in use. This method cannot be recommended for our case because the odor evolved during the process will become a nuisance to the nearby residents.
Control of Diseases in Animals [11] To prevent the diseases in animals from infection of coming air, the following measures are taken. The fresh air is filtered by passing through a glass fiber or mineral wool medium, which may be treated with disinfectant. One such type of filter which is commercially available is Permachem filter (Farm Filters Ltd., Treforest, U.K.) which is impregnated with bis (n-tributyl) tin oxide and general disinfectants. The filter can be relatively coarse because the infective particles can only survive in the air if protected by particles of dust. Treatment of Air Vented from the Building Even after implementing the steps #1 and #2 (as discussed above), the problem of odor may not be solved completely. Which practices of odor control will be most effective and economic in solving a given odor problem can never be predicted with absolute assurance. Often 'trial and error' procedures are necessary.
Counteraction and Masking Odor counteraction is the addition of one odor substance to another in such a way that the mixture has little or no odor. The Wolf Of Wall Street Torrent Download Kickass. Odor masking is the addition of an odorous substance, which, because of its characteristic and intensity, makes it impossible to detect the offensive odor. True cancellation is difficult, even when the characteristics of the offending odor are known. The use of deodorants actually means further pollution since the odors are only masked by another strongly smelling substance. It is better to use scrubbers, filters and combustion for the removal of odors (discussed later on). Use of Ozone Ozone is a strong oxidizing agent and may be used to oxidize some malodorous materials.
Ultraviolet lights are often used to produce ozone for the purpose of sweetening the air. The additional advantage of using ozone is the effect of inhibiting nasal sensory perception so those odors are not so readily detected. Miscellaneous Methods The absorbent that has found widest application in practice is activated carbon.
The activated carbon units are extremely effective in odor removal, if properly designed. Further, the odorous gas may be absorbed into liquids or solvents by the use of washers, condensers or scrubbers, (absorption process). For our case, for hydrogen sulfide-- an alkaline solution and for ammonia -- water, are quite effective.
These two gases are the main source of odor in livestock buildings. However, a combination of absorption--absorption method of odor removal is recommended. Such units are discussed by Pazar [13]. Physical Conditions In the prevention and control of odors, it is essential that careful consideration be given to meteorological, climatological and topographical conditions in the area. Location of livestock buildings at sites protected from wind by the topography should be avoided. Such conditions reduce air drainage, decrease the natural dilution of odors, and may lead to serious odor problems.
Special attention must be paid to the conditions such as warm climate, low winds, and frequent atmospheric inversions. These conditions are ripe for a serious odor problem. REFERENCES Albin, R. C., 'Handling and Disposal of Cattle Feedlot Waste,' Journal of Animal Science, Vol. 32, #4, 1971.
Phelps, 'Odor and Its Measurement,' Air Pollution, Vol. Stern (editor), (New York, Academic Press), pp. 305-327, 1968. R., 'Odor Control,' Journal of Water Pollution Control Federation, Vol. 583, April, 1972.
Jr., 'Hydrogen Sulfide Odor Control Measures,' Journal of Water Pollution Federation, Vol. Witheridge, Odors: Physiology and Control, (New York, McGraw Hill), 1949.
S., 'The Pollution Potential & Animal Wastes,' Agriculture Bulletin #15, Summer 1971. (Edmonton, The University of Alberta). Taiganides, E. P., 'Disposal of Animal Wastes,' Proceedings of the Nineteenth Industrial Waste Conference, May 5-7, 1964. (Indiana, Purdue University), Vol. Taiganides, E.P., T. Baumann, and H.
Johnson, 'Properties and Pumping Characteristics of Hog Wastes,' Transactions of the American Society of Agriculture Engineers, 7(2), 1964. E., 'Air Environment and Animal Performance,' Journal of Animal Science, Vol. 35, #3, Sept., 1972.